CHOLESTATIC PRURITUS
About
LIVMARLI
ROOT OUT
EXCESS BILE
What is LIVMARLI?
LIVMARLI is a medicine that is specifically designed to treat the itch in Alagille syndrome.
Who is LIVMARLI for?
LIVMARLI is FDA approved for the treatment of cholestatic pruritus (itch) in patients with Alagille syndrome who are 3 months of age and older.
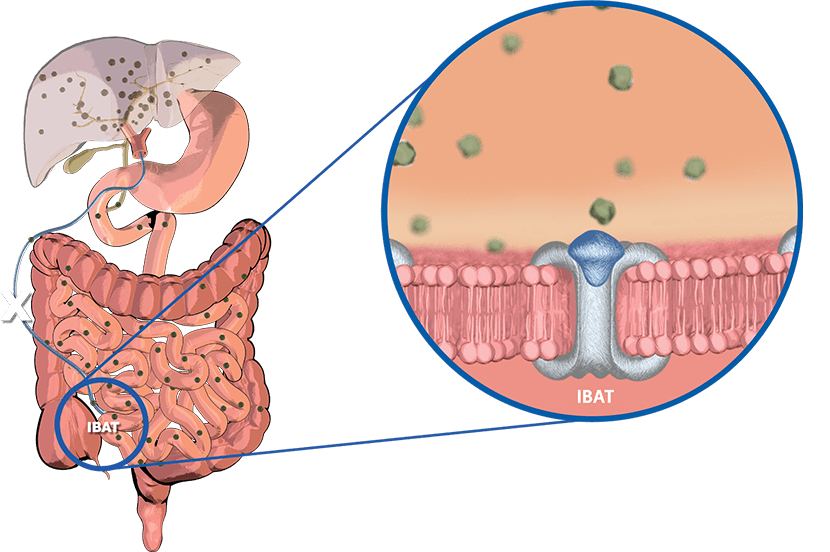
How does LIVMARLI work?
LIVMARLI targets and temporarily blocks something in your body called the ileal bile acid transporter (IBAT). In doing so, LIVMARLI lowers bile acids in the body (as measured by levels in the blood) by:
- Interrupting bile acids from going back into the liver
- Increasing the amount of bile acids that are removed from the body in feces
During the first year of treatment, more than 80% of patients experienced a decrease in bile acid buildup with LIVMARLI compared with when they started.
from going back into the liver.
- An FDA-approved treatment for the itch—also known as cholestatic pruritus—in patients with Alagille syndrome who are 3 months of age and older
- The way LIVMARLI improves itch is not completely known. It may involve inhibition of the IBAT, which interrupts bile acid recycling to the liver and decreases serum bile acids
- In the clinical study for LIVMARLI, reductions in bile acid buildup were associated with decreases in itch intensity
SEE HOW LIVMARLI WORKS IN THE BODY
[00:00:00]
Let's take a closer look at bile acid buildup in Alagille syndrome. In the body, a fluid called bile helps break down fat in the food we eat. Bile acids are a part of bile. Normally, bile is made in the liver and then moves to the gallbladder for storage. When food enters the small intestine, the bile is then sent in to help with digestion and break down fat. Almost [00:00:30] all of the bile and bile acids made by the liver are recycled.
This means they return to the liver to be used again. In the intestine, the ileal bile acid transporter or IBAT, is responsible for bile acid recycling. Bile acids that are not recycled are removed from the body in feces. They're then replaced by new bile acids in the liver. In Alagille syndrome, the flow of bile out of the liver is impaired. This leads [00:01:00] to a buildup of bile acids in the liver. Some of the bile acids can also get into the blood. This can cause an increase in bile acids throughout the entire body.
In Alagille syndrome, the buildup of bile acids in the liver and blood occurs because of a problem with the tubes called bile ducts that allow bile to flow out of the liver. This buildup of bile acids in the liver may cause liver damage and other problems. The most troublesome problem is often a severe itch, also known as cholestatic pruritus. With LIVMARLI, you can [00:01:30] battle bile acid buildup. A treatment for people with the itch in Alagille syndrome who are 3 months of age and older—
[music]
—and the first FDA-approved medicine for the itch in Alagille syndrome. LIVMARLI inhibits the IBAT and prevents the recycling of bile acids back to the liver. By interrupting the flow of [00:02:00] bile acids in this way, LIVMARLI increases the amount of bile acids that are removed from the body in feces, and lowers bile acid levels in the body. Although the way by which LIVMARLI improves itch is not completely known, it may involve this decrease in bile acid buildup that occurs with inhibition of the IBAT. In a clinical study, a reduction in bile acid buildup was associated with reductions in itch intensity.
[00:02:30] INDICATION. LIVMARLI is a prescription medicine used to treat cholestatic pruritus (itch) in patients who are 3 months of age and older with Alagille syndrome.
It is not known if LIVMARLI is safe and effective in children with Alagille syndrome who are under 3 months of age. It is not known if LIVMARLI is safe and effective in adults who are 65 years of age and older.
IMPORTANT SAFETY INFORMATION
What are the possible side effects of LIVMARLI?
LIVMARLI can cause serious side effects, including:
- Liver injury. Changes in certain liver tests are common in patients but may worsen during treatment with LIVMARLI. These changes may be a sign of liver injury and can be serious. Your health care provider should do blood tests and physical exams before starting and during treatment to check your liver function. Tell your health care provider right away if you get any signs or symptoms of liver problems, including:
- nausea or vomiting
- your skin or the white part of your eye turns yellow
- dark or brown urine
- pain on the right side of your stomach (abdomen)
- fullness, bloating, or fluid in your stomach area (ascites)
- loss of appetite
- bleeding or bruising more easily than normal, including vomiting blood
- Stomach and intestinal (gastrointestinal) problems. LIVMARLI can cause stomach and intestinal problems, including diarrhea and stomach pain during treatment. Diarrhea can also cause the loss of too much body fluid (severe dehydration). Your health care provider may advise you to monitor for new or worsening stomach problems, including stomach pain, diarrhea, blood in your stool, or vomiting.
Tell your health care provider right away if you have any new or worsening signs or symptoms of stomach and intestinal problems, including:- diarrhea
- more frequent bowel movements than usual
- stools that are black, tarry, or sticky, or have blood or mucous
- severe stomach-area pain or tenderness
- vomiting
- urinating less often than usual
- dizziness
- headache
- A condition called Fat-Soluble Vitamin (FSV) Deficiency caused by low levels of certain vitamins (vitamin A, D, E, and K) stored in body fat. FSV deficiency is common in patients with Alagille syndrome but may worsen during treatment. Your health care provider should do blood tests before starting and during treatment, and may monitor for bone fractures and bleeding, which are common side effects
Tell your health care provider about all medicines that you take, as LIVMARLI may interact with other medicines. If you take a medicine that lowers cholesterol by binding bile acids, such as cholestyramine, colesevelam, or colestipol, take it at least 4 hours before or 4 hours after you take LIVMARLI.
Your health care provider may change your dose, or temporarily or permanently stop treatment if you have certain side effects.
LIVMARLI is taken by mouth, 1 time each day, 30 minutes before a meal in the morning. Be sure to use the provided oral dosing dispenser to accurately measure the dose of medicine.
These are not all of the possible side effects of LIVMARLI. For more information, ask your health care provider or pharmacist. Call your doctor for medical advice about side effects. You may report side effects to the FDA at 1-800-FDA-1088.
It's time for people with Alagille syndrome to take aim against bile acid buildup. It's time for LIVMARLI.
[music]
[00:04:58] [END OF AUDIO]

Always
Safety
First!

Is LIVMARLI safe?
Backed by more than 5 years of safety data,* LIVMARLI has a well-known safety profile in patients with Alagille syndrome.
*From extended observation of patients taking LIVMARLI after completing a placebo-controlled study.
LIVMARLI has a well-known safety record in patients 3 months to 12 months of age, similar to patients 1 year of age and older.
What are the possible side effects?
LIVMARLI may cause side effects. Talk to your doctor or your child’s doctor about what to expect and how to manage any side effects.
The most common side effects with LIVMARLI include:
Liver injury. Changes in certain liver tests are common in patients but may worsen during treatment with LIVMARLI. These changes may be a sign of liver injury and can be serious. Your health care provider should do blood tests and physical exams before starting and during treatment to check your liver function. Tell your health care provider right away if you get any signs or symptoms of liver problems, including:
- nausea or vomiting
- your skin or the white part of your eye turns yellow
- dark or brown urine
- pain on the right side of your stomach (abdomen)
- fullness, bloating, or fluid in your stomach area (ascites)
- loss of appetite
- bleeding or bruising more easily than normal, including vomiting blood
Stomach and intestinal (gastrointestinal) problems. LIVMARLI can cause stomach and intestinal problems, including diarrhea and stomach pain during treatment. Diarrhea can also cause the loss of too much body fluid (severe dehydration). Your health care provider may advise you to monitor for new or worsening stomach problems, including stomach pain, diarrhea, blood in your stool, or vomiting. Tell your health care provider right away if you have any new or worsening signs or symptoms of stomach and intestinal problems, including:
- diarrhea
- more frequent bowel movements than usual
- stools that are black, tarry, or sticky, or have blood or mucous
- severe stomach-area pain or tenderness
- vomiting
- urinating less often than usual
- dizziness
- headache
A condition called Fat-Soluble Vitamin (FSV) Deficiency caused by low levels of certain vitamins (vitamin A, D, E, and K) stored in body fat. FSV deficiency is common in patients with Alagille syndrome but may worsen during treatment. Your health care provider should do blood tests before starting and during treatment, and may monitor for bone fractures and bleeding, which are common side effects
Your health care provider may change your dose, or temporarily or permanently stop treatment if you have certain side effects.
These are not all of the possible side effects of LIVMARLI. For more information, ask your health care provider or pharmacist. Call your doctor for medical advice about side effects. You may report side effects to the FDA at 1-800-FDA-1088.
LIVMARLI can be taken even if you have other medical conditions.

Talk to Your Doctor About LIVMARLI
Get the conversation started about whether LIVMARLI is right for you or your child.


Lower Costs Within Reach
Mirum Access Plus works closely with your doctor and insurance plan to help facilitate coverage for LIVMARLI.
Learn How to Enroll
