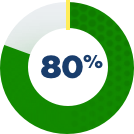
How was the impact on the liver studied?
There were 3 clinical studies conducted for patients with Alagille syndrome. Some patients from those studies chose to stay on LIVMARLI long term. These patients were observed to see if there were any factors that could predict transplant-free survival.
Patients were included if they had moderate-to-severe itch, were considered to be benefitting from LIVMARLI, stayed on treatment for at least 48 weeks, and had lab results at 48 weeks. All patients took LIVMARLI.